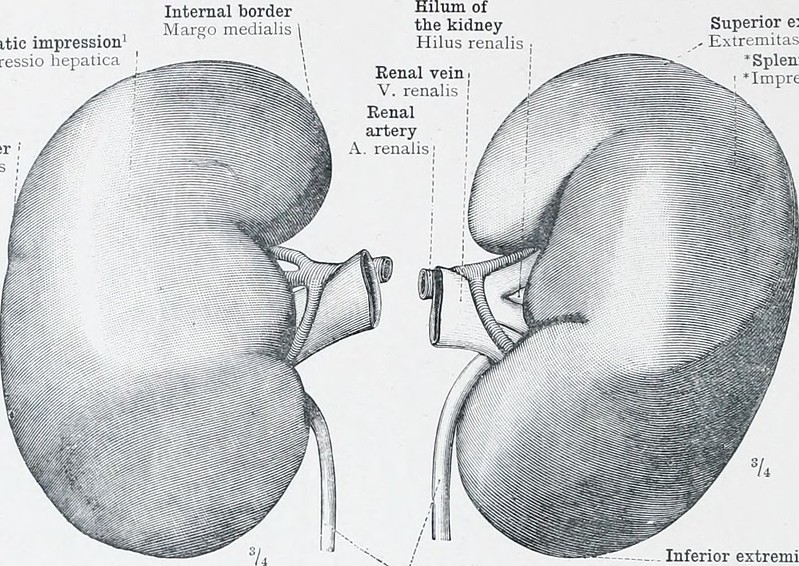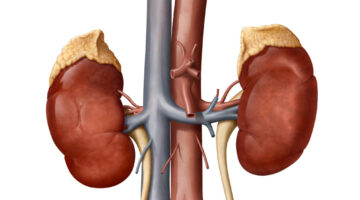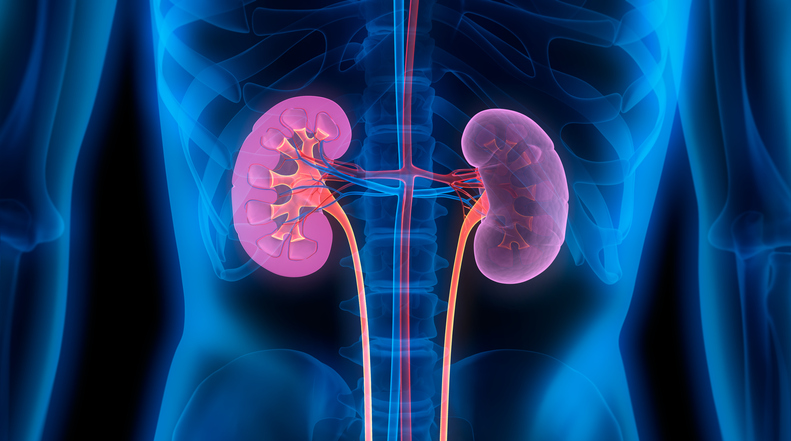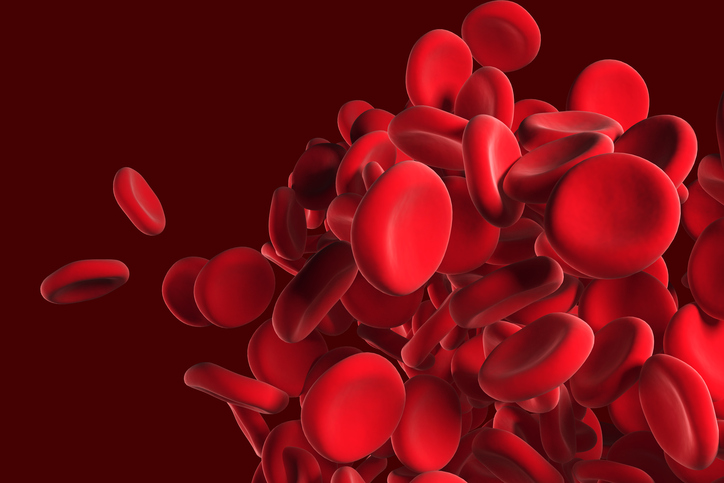
Novo Nordisk Gets New Complement System Approach to Rare Diseases With Deal for Omeros Drug
Novo Nordisk is paying $240 million up front for global rights to zaltenibart, an Omeros drug that blocks the MASP-3 protein to treat diseases caused by excessive activity of the complement system. This antibody drug is on track for pivotal testing that could show superiority over blockbuster AstraZeneca drugs that are standard treatments for a rare blood disorder.